产品
编 号:F098128
分子式:C25H20F2N2O2S
分子量:450.5
分子式:C25H20F2N2O2S
分子量:450.5
产品类型
规格
价格
是否有货
结构图
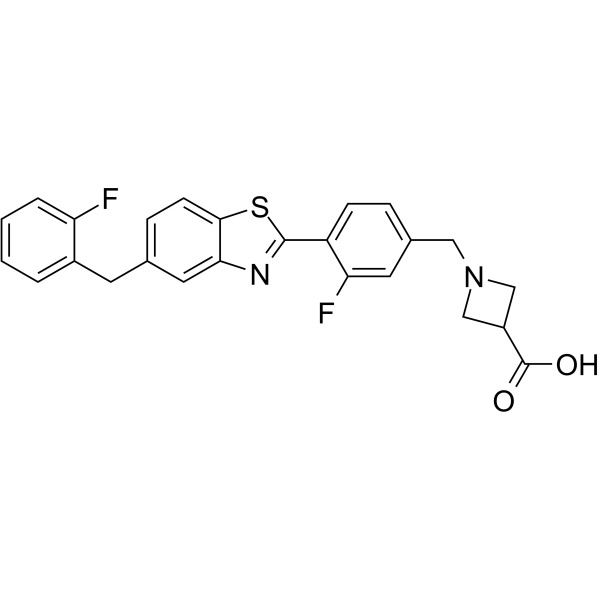
CAS No: 1257093-40-5
产品详情
生物活性:
TC-SP 14 (compound 14) is an orally active and potent S1P1 agonist (EC50 = 0.042 μM) with minimal activity at S1P3 (EC50 = 3.47 μM). TC-SP 14 significantly reduces blood lymphocyte counts and attenuates a delayed type hypersensitivity (DTH) response to antigen challenge.
体内研究:
TC-SP 14 (compound 14) (0-3 mg/kg, Orally, once) produces a dose-dependent reduction in circulating blood lymphocytes 24 h postdose.TC-SP 14 (0-3 mg/kg, Orally, daily for 10 days) significant reduces ovalbumin (OVA)-induced ear swelling.TC-SP 14 (2-15 mg/kg, IV or PO, once) possesses acceptable characteristics.Pharmacokinetic Parameters of TC-SP 14 in female Sprague-Dawley rats and male Cynomolgus .speciesrat NHP
CL (L/h/kg)0.330.50
Vss (L/kg)3.31.6
T1/2 (h)7.535.2
MRT (h)103.3
% F6823
Animal Model:Lewis rats (female, n = 5/group)
Dosage:0.3, 1.0, and 3.0 mg/kg
Administration:Orally, once
Result:Produced a dose-dependent reduction in circulating blood lymphocytes 24 h postdose, resulted in near maximal lymphopenia at 3.0 mg/kg (74% reduction in lymphocytes vs vehicle).
Animal Model:OVA-immunized Lewis rats (female, n = 8/group)
Dosage:0.1, 0.3, 1.0, and 3.0 mg/kg
Administration:Orally, daily for 10 days
Result:Significant reduced OVA-induced ear swelling at doses of 0.3 mg/kg and higher.
Animal Model:Female Sprague-Dawley rats, Male Cynomolgus (NHP (nonhuman primates)) (n=3/group)
Dosage:2 (IV, rat), 4 (IV, NHP), 10 (PO, NHP), 15 mg/kg (PO, rat)
Administration:IV, PO, once (Pharmacokinetic Analysis)
Result:Possessed acceptable characteristics, demonstrated low clearance, moderate steady state volumes of distribution, moderate-to-long mean residence times, and acceptable oral bioavailability.
体外研究:
TC-SP 14 (compound 14) neither inhibits nor induces human cytochrome P450 enzymes, is nonmutagenic, and dose not significantly inhibit the hERG channel.
TC-SP 14 (compound 14) is an orally active and potent S1P1 agonist (EC50 = 0.042 μM) with minimal activity at S1P3 (EC50 = 3.47 μM). TC-SP 14 significantly reduces blood lymphocyte counts and attenuates a delayed type hypersensitivity (DTH) response to antigen challenge.
体内研究:
TC-SP 14 (compound 14) (0-3 mg/kg, Orally, once) produces a dose-dependent reduction in circulating blood lymphocytes 24 h postdose.TC-SP 14 (0-3 mg/kg, Orally, daily for 10 days) significant reduces ovalbumin (OVA)-induced ear swelling.TC-SP 14 (2-15 mg/kg, IV or PO, once) possesses acceptable characteristics.Pharmacokinetic Parameters of TC-SP 14 in female Sprague-Dawley rats and male Cynomolgus .speciesrat NHP
CL (L/h/kg)0.330.50
Vss (L/kg)3.31.6
T1/2 (h)7.535.2
MRT (h)103.3
% F6823
Animal Model:Lewis rats (female, n = 5/group)
Dosage:0.3, 1.0, and 3.0 mg/kg
Administration:Orally, once
Result:Produced a dose-dependent reduction in circulating blood lymphocytes 24 h postdose, resulted in near maximal lymphopenia at 3.0 mg/kg (74% reduction in lymphocytes vs vehicle).
Animal Model:OVA-immunized Lewis rats (female, n = 8/group)
Dosage:0.1, 0.3, 1.0, and 3.0 mg/kg
Administration:Orally, daily for 10 days
Result:Significant reduced OVA-induced ear swelling at doses of 0.3 mg/kg and higher.
Animal Model:Female Sprague-Dawley rats, Male Cynomolgus (NHP (nonhuman primates)) (n=3/group)
Dosage:2 (IV, rat), 4 (IV, NHP), 10 (PO, NHP), 15 mg/kg (PO, rat)
Administration:IV, PO, once (Pharmacokinetic Analysis)
Result:Possessed acceptable characteristics, demonstrated low clearance, moderate steady state volumes of distribution, moderate-to-long mean residence times, and acceptable oral bioavailability.
体外研究:
TC-SP 14 (compound 14) neither inhibits nor induces human cytochrome P450 enzymes, is nonmutagenic, and dose not significantly inhibit the hERG channel.
产品资料

