产品
编 号:F620984
分子式:C44H87NO5
分子量:710.17
分子式:C44H87NO5
分子量:710.17
产品类型
规格
价格
是否有货
5mg
640
In-stock
10mg
960
In-stock
25mg
1760
In-stock
50mg
2560
In-stock
100mg
3680
In-stock
结构图
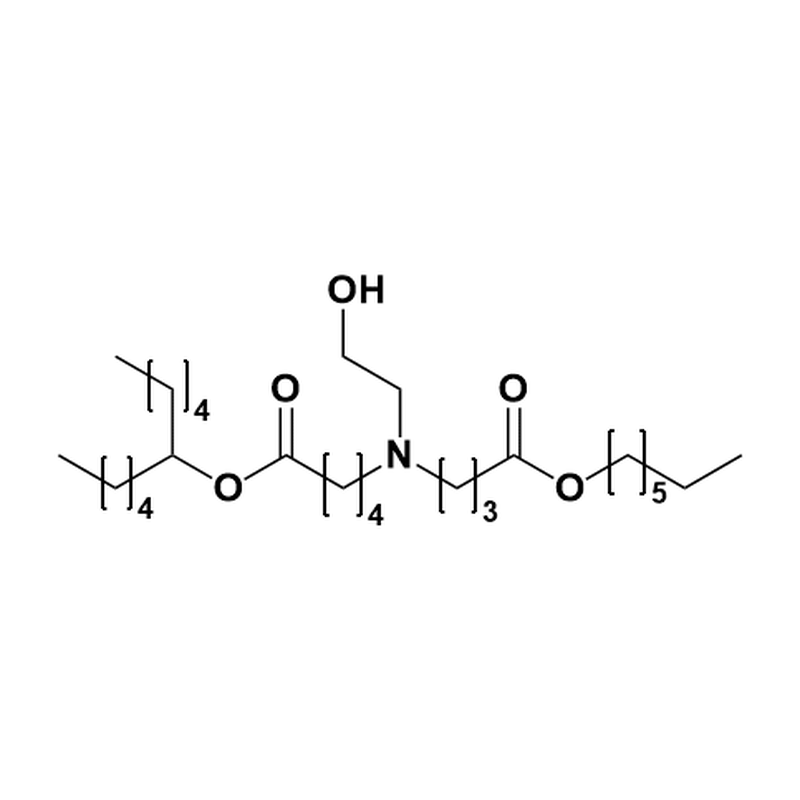
CAS No: 2089251-47-6
产品详情
产品资料

