产品
编 号:F027255
分子式:C32H43N5O4
分子量:561.71
分子式:C32H43N5O4
分子量:561.71
产品类型
规格
价格
是否有货
10mM*1mL in DMSO
询价
询价
1mg
询价
询价
5mg
询价
询价
10mg
询价
询价
25mg
询价
询价
50mg
询价
询价
结构图
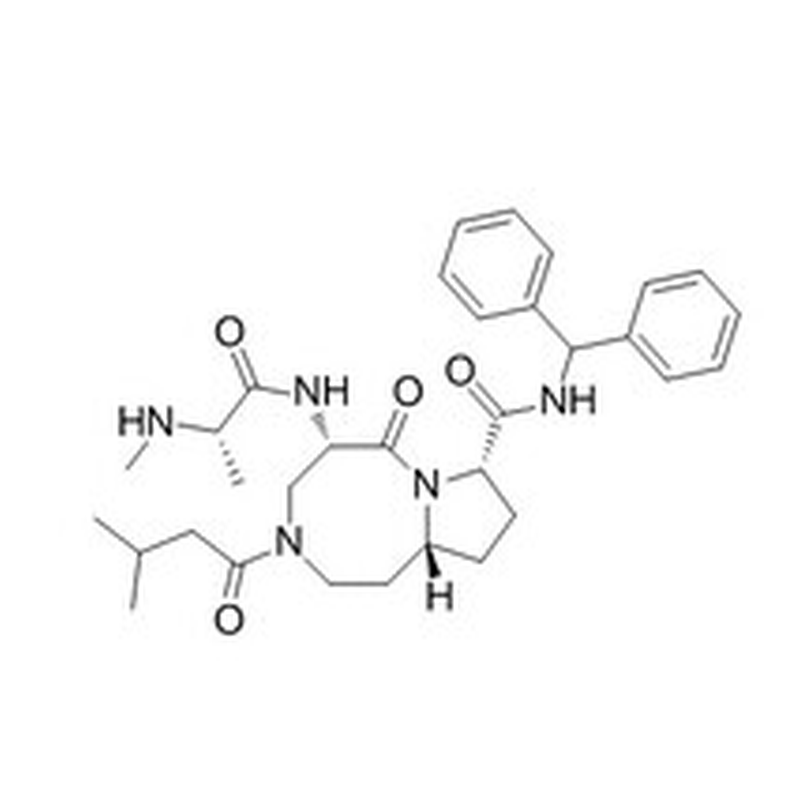
CAS No: 1071992-99-8
产品详情
生物活性:
Xevinapant (AT-406) is a potent and orally bioavailable Smac mimetic and an antagonist of IAPs, and it binds to XIAP, cIAP1, and cIAP2 proteins with Ki of 66.4, 1.9, and 5.1 nM, respectively.
体内研究:
Xevinapant (AT-406) is very effective in inhibition of tumor growth in the MDA-MB-231 xenograft model, and has minimal toxicity to animals. Xevinapant is evaluated for its pharmacokinetic (PK) properties in mice, rats, non-human primates and dogs.Animal Model:SCID mice bearing MDA-MB-231 xenograft tumors
Dosage:30 and 100 mg/kg
Administration:p.o.; 5 days a week for 2 weeks
Result:Strongly inhibits tumor growth at 30 and 100 mg/kg and completely inhibits tumor growth during the treatment with 100 mg/kg.
体外研究:
Xevinapant mimic closely the AVPI peptide in both hydrogen bonding and hydrophobic interactions with XIAP, with additional hydrophobic contacts with W323 of XIAP. Xevinapant is more sensitive to these IAPs than Smac AVPI peptide with 50-100 fold binding affinities. Xevinapant (1 μM) completely restores the activity of caspase-9, which is suppressed by 500 nM XIAP BIR3 in a cell-free system. In MDA-MB-231 cell, Xevinapant induces rapid cellular cIAP1 degradation and also pulls down the cellular XIAP protein. Xevinapant effectively inhibits lots of human cancer cell lines and shows IC50 of 144 and 142 nM in MDA-MB-231 cell and SK-OV-3 ovarian cell, with low toxicity against normal-like human breast epithelial MCF-12F cells and primary human normal prostate epithelial cells. Xevinapant induces apoptosis in MDA-MB-231 cell by inducing activation of caspase-3 and cleavage of PARP. Xevinapant displays single agent activity in ovarian cancer cell lines. The IC50 values of AT-406 in these ovarian cancer cells range from 0.05-0.5 μg/mL. Xevinapant exhibits anti-ovarian cancer efficacy both as a single agent and in combination with carboplatin. Xevinapant (30 μg/mL) induced degradation of XIAP in the drug sensitive ovarian cancer cell lines.
Xevinapant (AT-406) is a potent and orally bioavailable Smac mimetic and an antagonist of IAPs, and it binds to XIAP, cIAP1, and cIAP2 proteins with Ki of 66.4, 1.9, and 5.1 nM, respectively.
体内研究:
Xevinapant (AT-406) is very effective in inhibition of tumor growth in the MDA-MB-231 xenograft model, and has minimal toxicity to animals. Xevinapant is evaluated for its pharmacokinetic (PK) properties in mice, rats, non-human primates and dogs.Animal Model:SCID mice bearing MDA-MB-231 xenograft tumors
Dosage:30 and 100 mg/kg
Administration:p.o.; 5 days a week for 2 weeks
Result:Strongly inhibits tumor growth at 30 and 100 mg/kg and completely inhibits tumor growth during the treatment with 100 mg/kg.
体外研究:
Xevinapant mimic closely the AVPI peptide in both hydrogen bonding and hydrophobic interactions with XIAP, with additional hydrophobic contacts with W323 of XIAP. Xevinapant is more sensitive to these IAPs than Smac AVPI peptide with 50-100 fold binding affinities. Xevinapant (1 μM) completely restores the activity of caspase-9, which is suppressed by 500 nM XIAP BIR3 in a cell-free system. In MDA-MB-231 cell, Xevinapant induces rapid cellular cIAP1 degradation and also pulls down the cellular XIAP protein. Xevinapant effectively inhibits lots of human cancer cell lines and shows IC50 of 144 and 142 nM in MDA-MB-231 cell and SK-OV-3 ovarian cell, with low toxicity against normal-like human breast epithelial MCF-12F cells and primary human normal prostate epithelial cells. Xevinapant induces apoptosis in MDA-MB-231 cell by inducing activation of caspase-3 and cleavage of PARP. Xevinapant displays single agent activity in ovarian cancer cell lines. The IC50 values of AT-406 in these ovarian cancer cells range from 0.05-0.5 μg/mL. Xevinapant exhibits anti-ovarian cancer efficacy both as a single agent and in combination with carboplatin. Xevinapant (30 μg/mL) induced degradation of XIAP in the drug sensitive ovarian cancer cell lines.
产品资料

